Geraint Williams, ALS Laboratories and Tim Rolfe, YES Engineering, both members of the AGS Contaminated Working Group, discuss how representative samples should be collected to ensure subsequent laboratory analysis is robust and reliable.
Introduction
This article covers the preservation requirements for common contaminants and key chemical indicators of Monitored Natural Attenuation (MNA) including metals, ammoniacal nitrogen, cyanide, sulphide and manganese II. It provides practical guidance for those involved in groundwater monitoring and surface water sampling and is relevant for risk assessors, remediation contractors and regulators.
In September 2022, members of the contaminated land group were invited to complete a short questionnaire. This survey highlighted inconsistencies in the use of chemical preservation. AGS received comments about a lack of confidence in laboratory results where preservation has not been used, the need to follow established practice and a call for more scrutiny and oversight: “I would have no confidence in the data…”. Other direct quotes include: “we review reports where preservation has not been used and have to conduct further rounds of monitoring to obtain better quality data”.
Taking account of the concerns highlighted in the previous survey, the following article provides more information on why preservation is so important.
Dissolved Metals
Dissolved metals can be impacted by many physical and chemical factors, particularly redox conditions, pH or temperature which can trigger changes due to precipitation, co-precipitation, sorption or dissolution of particulate matter. These factors can cause significant positive or negative bias to dissolved metal concentrations.
Samples are acidified to prevent precipitation of metals, especially iron. Once precipitation has occurred there is no way of knowing how much metal was in solution, or in suspension, at the time of sampling. The only way of resolving this is to filter out suspended metals in the field, placing the filtered sample in a dedicated nitric acid bottle to ensure that all metal dissolved at the time of sampling remains in solution.
Ferrous Iron
Ferrous iron, once sampled, will generally rapidly oxidise to ferric iron and precipitate as ferric oxyhydroxide. Hydrochloric acid is used to fix the ratio of ferrous and ferric iron. Adding the acid in the laboratory is not an acceptable substitute since the ferrous iron is highly likely to have oxidised in transport.
The concentrations of ferrous and ferric iron are used as supporting evidence for the presence of MNA of organic contaminants by biodegradation, therefore measurement of the correct species is essential to understanding the aquifer conditions.
When iron precipitation occurs in a sample, other metals can co-precipitate, causing substantial changes to the overall dissolved composition of metals. Co-precipitation of metals is covered in more detail in a previous AGS magazine article published back in August 2020. It highlights how concentrations of arsenic, lead and cadmium can be significantly affected with losses of between 80 to 97% reported.
Manganese II
Samples for manganese II require filtering in the field to remove insoluble Mn IV compounds before adding to a bottle containing hydrochloric acid. The acid prevents oxidation of Mn II to insoluble Mn IV. Mn II acts as an indicator of anaerobic degradation of organics, where manganese IV acts as an electron acceptor.
Ammoniacal Nitrogen
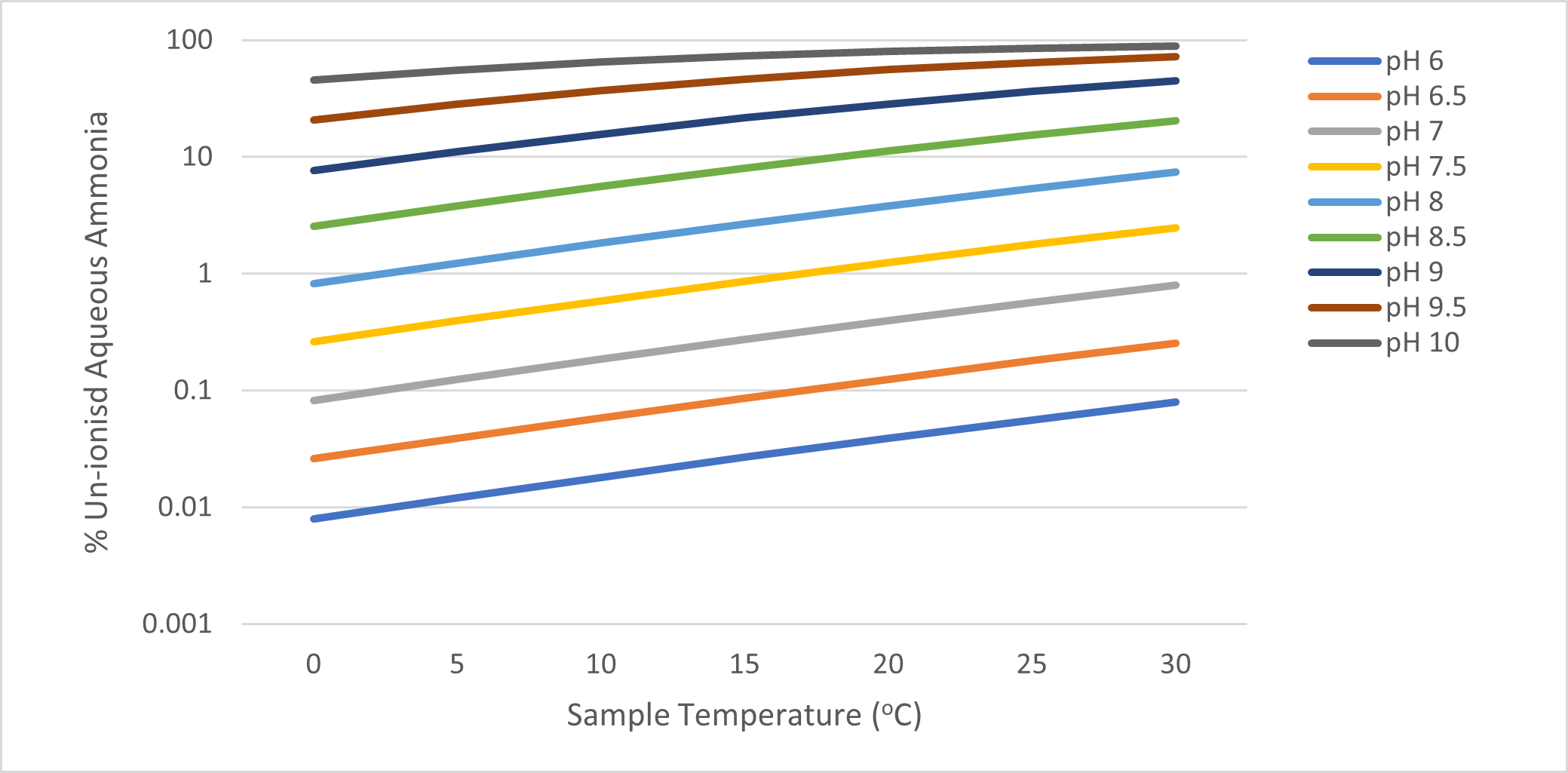
Ammoniacal nitrogen includes both the ionised form (ammonium, NH4+) and the unionised form (ammonia, NH3). An increase in pH favours formation of the more toxic unionised form (NH3), while a decrease favours the ionised (NH4+) form. Temperature also affects the toxicity and form of ammonia. This relationship is detailed in the Table 1.
Table 1 Percentage Un-ionised Aqueous Ammonia (0-30°C, pH 6-10)
Source: Canadian Council of Ministers of the Environment (CCME) (2010) Canadian Water Quality Guidelines for the Protection of Aquatic Life.
It is possible to simply measure the ammoniacal nitrogen, and then to calculate the ammonia, if the pH and temperature of the sample were measured at source. As illustrated above, at the range of pH typically encountered in groundwater samples, the percentage of the more toxic un-ionised ammonia can increase approximately threefold between a sample temperature of 10oC and a room temperature in the laboratory of 20oC.
Because of the volatility of ammonia, the action of nitrifying bacteria, and the changing equilibrium between ammonia and ammonium, groundwater and surface water samples must be collected using sulphuric acid to fix the ammoniacal compounds to prevent further change. Sulphuric acid reduces pH to <2. The acid will convert the ammonia to ammonium and the results are reported as ammoniacal nitrogen. This prevents microbial degradation and off-gassing of ammonia.
Cyanide
Laboratories use sodium hydroxide to keep the water alkaline and the cyanide in solution. If the water is not preserved and is slightly acidic, the cyanide may convert to hydrogen cyanide and be lost from the sample. The reported concentration from the laboratory will therefore underestimate the cyanide present in groundwater or surface water, and where speciated cyanide analysis is being undertaken the concentration of the more toxic ‘free-cyanide’ will be most affected.
Sulphide
Sulphide oxidises to sulphate in contact with oxygen. The industry standard technique for preservation of sulphide utilises zinc acetate. Zinc ions from zinc acetate react rapidly to form zinc sulphide, an insoluble precipitate, sequestering the sulphide species and preventing off-gassing and oxidation. Ensuring that the correct proportions of sulphide and sulphate are reported is essential to assessments of concrete design classification and of aquifer conditions for MNA decisions.
Summary
How samples are collected and analysed is crucial to the reliability of human health and controlled water risk assessment and in assessing the effectiveness of MNA. Chemical preservation is required to ensure that samples are representative of field conditions. Samples collected for analysis of unstable contaminants, that have not been preserved, are unlikely to provide valid, consistent, or defensible analytical data. This article is written in advance of guidance being published by Contaminated Land: Applications in Real Environments (CL:AIRE) on MNA.
References
- AGS Guide to Environmental Sampling, Association of Geotechnical and Geoenvironmental Specialists, 2019
- BS EN ISO 5667-3: 2018 Water quality: Sampling – Part 3: Guidance on the preservation and handling of samples
- BS ISO 5667-11: 2009 Water quality. Sampling. Guidance on sampling groundwaters
- CCME (2010) Canadian Water Quality Guidelines for the Protection of Aquatic Life. Publication No. 1299; ISBN 1-896997-34-1
- Society of Brownfield Risk Assessment (SoBRA) Practical Tips to Share: Improving Risk Assessment – Field to Desk
Image credit to ALS Laboratories (UK) Limited
Article provided by Geraint Williams, ALS Laboratories and Tim Rolfe, YES Engineering